how many valence electrons does bromine have|How Many Valence Electrons Does Bromine (Br) : iloilo If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains . Paito Warna Macau, Data Warna Atau Paito Togel Macau terbaru tarikan warna warni toto untuk merumus jitu togel master.Paito Macau saat ini sangat berguna dan banyak dicari oleh pecinta togel Macau yang ingin mengetahui pola tentang pengeluaran togel Macau dari data Macau yang muncul tiap hari.
PH0 · Valences of the Chemical Elements
PH1 · Khan Academy
PH2 · How to Find the Valence Electrons for Bromine (Br)
PH3 · How many valence electrons does bromine have?
PH4 · How many valence electrons does Bromine have?
PH5 · How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bro
PH6 · How Many Valence Electrons Does Bromine (Br) Have?
PH7 · How Many Valence Electrons Does Bromine (Br)
PH8 · Complete Electron Configuration for Bromine (Br, Br
PH9 · Bromine
At Holina Rehab, our holistic approach addresses the six primary human needs—certainty, significance, variety, love/connection, growth, and contribution—creating a comprehensive and healthy framework for recovery. This approach allows us to effectively tackle the full range of symptoms related to addiction and trauma.
how many valence electrons does bromine have*******Set 2, 2020 — There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine has in its valence shell. To do so,.
The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven valence electrons. .
Bromine is the third halogen, being a nonmetal in group 17 of the periodic table. Its properties are thus similar to those of fluorine, chlorine, and iodine, and tend to be intermediate between those of the two neighbouring halogens, chlorine, and iodine. Bromine has the electron configuration [Ar]4s 3d 4p , with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising age.
Peb 3, 2021 — Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valence electrons of bromine are seven and the valency is one, based on its electron .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains .93 rows — May 19, 2024 — You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be .
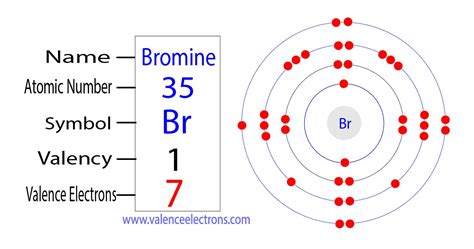
Mar 18, 2023 — The electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of bromine are seven. The elements that have 5, 6, or 7 electrons in the .
Carbon, sulfur, nitrogen, calcium, magnesium, sodium, maybe ten other elements are also involved in life, but none of them have the power of iron to move electrons around, and .May 6, 2018 — 7 only the electrons in the outmost shell are valance electrons.All but seven of the electrons in bromine are in lower shells Bromine is in family VII A. the same as Fluorine Chlorine. All members of the family have .Ago 22, 2024 — How many electrons does a bromine atom have? Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. . Also, the .
How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.May 19, 2024 — This table of element valences includes the maximum valence and most common valence values in chemistry. . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking . Bromine-1, +1, (+3), (+4), +5: 36: Krypton: 0: 37: Rubidium .
Dis 15, 2019 — If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and .
Bromine has an electron configuration of $$1s^{2}\ 2s^{2}\ 2p^{6}\ 3s^{2}\ 3p^{6}\ 4s^{2}\ 3d^{10}\ 4p^{5} $$ the valence electrons are in the $$4s$$ and $$4p$$ orbitals giving Bromine $$7$$ valence electrons.You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons, respectively. Valence electrons are responsible for the reactivity of an element.Mar 3, 2021 — There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 4p 5. The valence electrons are be the 4s and 4p electrons. Bromine has seven valence electrons.How many valence electrons does Bromine have? There are 2 steps to solve this one. Step 1. Concept : Valance electrons : Valence electrons are those electrons which are present in the outermo. View the full answer. Step 2. Unlock. Answer. Unlock. Previous question Next question.Study with Quizlet and memorize flashcards containing terms like According to the periodic table, how many electrons does bromine (#35) have in its valence level?, Calcium and bromine have formed a bond. Leading up to this, calcium gave up electrons. It was a(n) ____., Which of the following are not likely to form bonds? and more.Abr 17, 2023 — For example, silicon is in Group IVA (Group 14), so each atom would have four valence electrons. Chlorine is in Group VIIA (Group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in Group IIA (Group 2). Helium is the only exception for the main group elements. The first energy .
how many valence electrons does bromine haveHul 20, 2023 — Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty-five protons and forty-four neutrons in its nucleus, and thirty-five electrons in four shells. It is located in group seventeen, period four and block p of the periodic .How Many Valence Electrons Does Bromine (Br) Bromine (Br) is a halogen and has an atomic number of 35. It has electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Bromine has 7 valence electrons and 4 energy levels.
A bromine (Br) atom has 7 valence electrons. Valence electrons are the outermost electrons in an atom, which affect how atoms might react with one.

Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell. [30]
Another way to find the valence electrons is by the group number the atom is found in.Br is in group 7A of the periodic table, which means it has 7 valence electrons (as all other atoms in the same group).A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are abundant, making up more than half of all visible matter.It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron.The neutron has a mean square .
Cleaning an IPS monitor is essential to maintain its longevity and performance. To clean an IPS monitor, you will need the following equipment: Microfiber cloth: A microfiber cloth is a soft and delicate cloth ideal for cleaning an IPS monitor’s screen. It is gentle on the surface and leaves no scratches or marks.
how many valence electrons does bromine have|How Many Valence Electrons Does Bromine (Br)